Platform
It's All about the Antibody Glycan
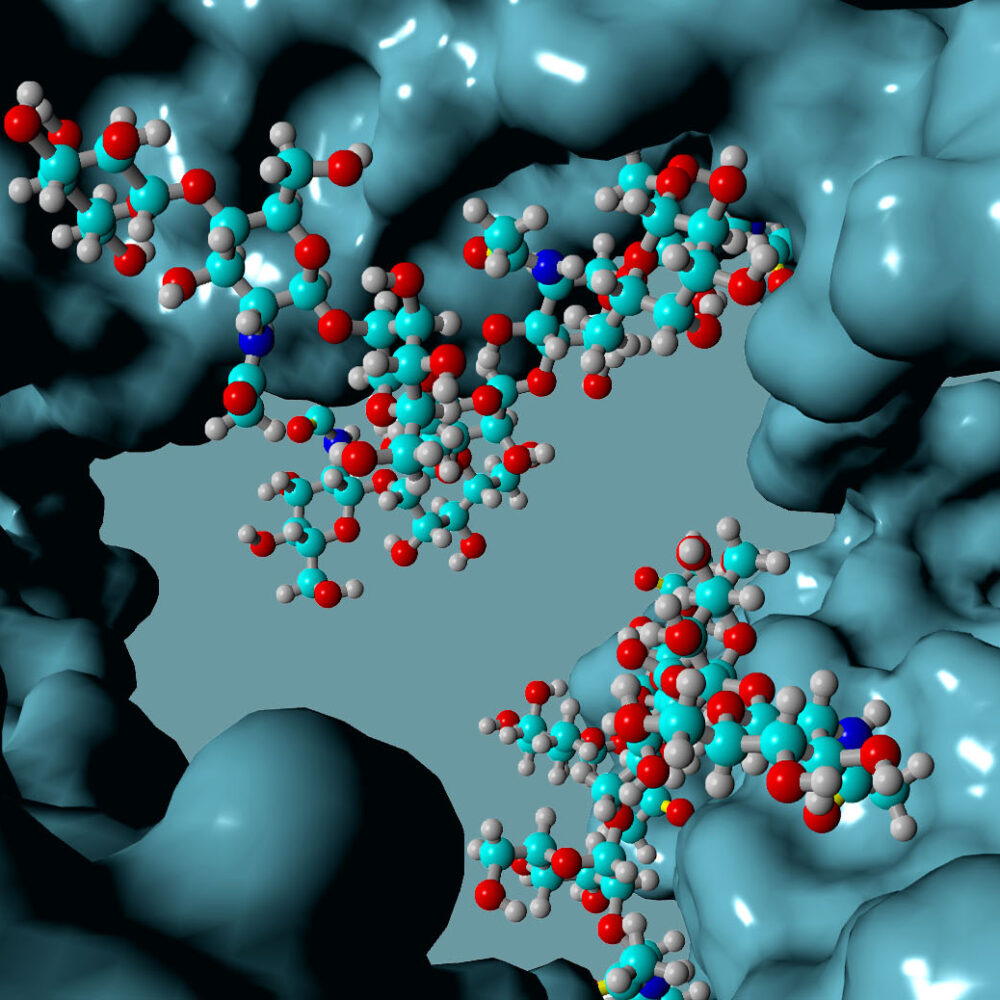
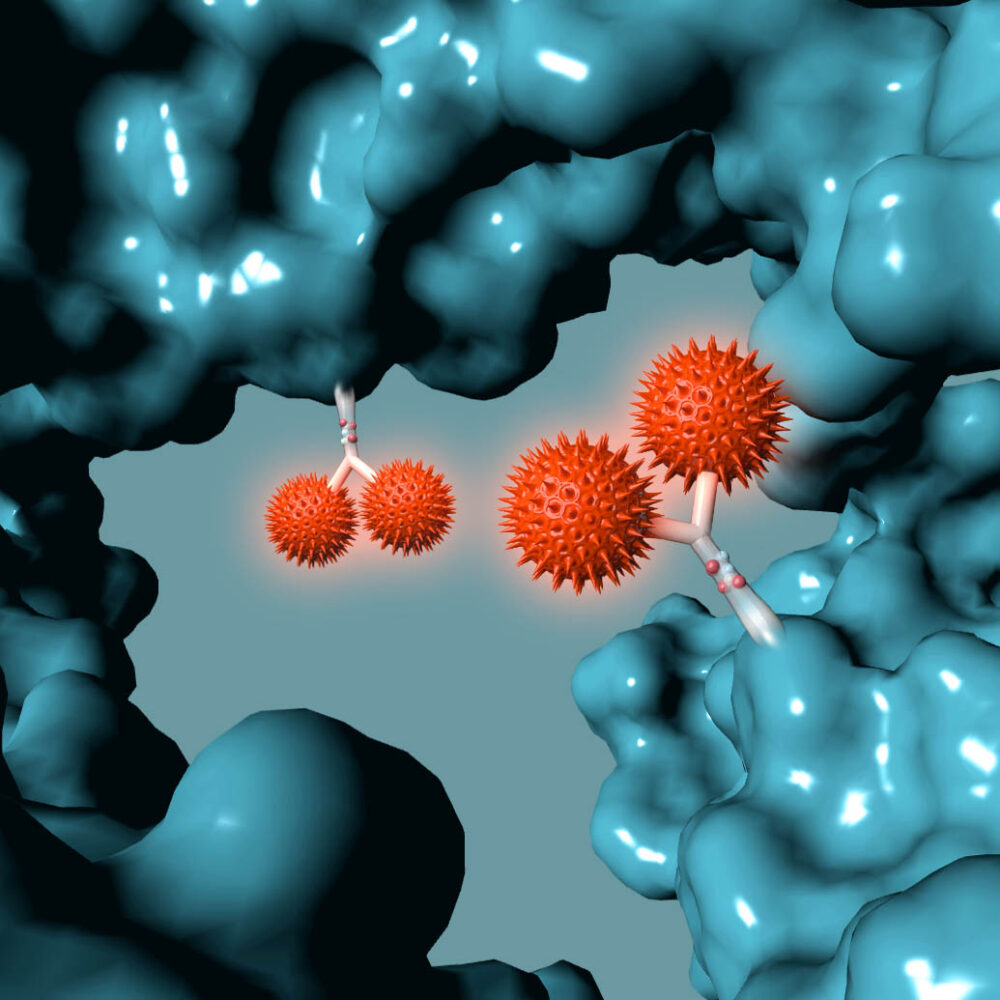
Drug Antibody Ratio 4:1
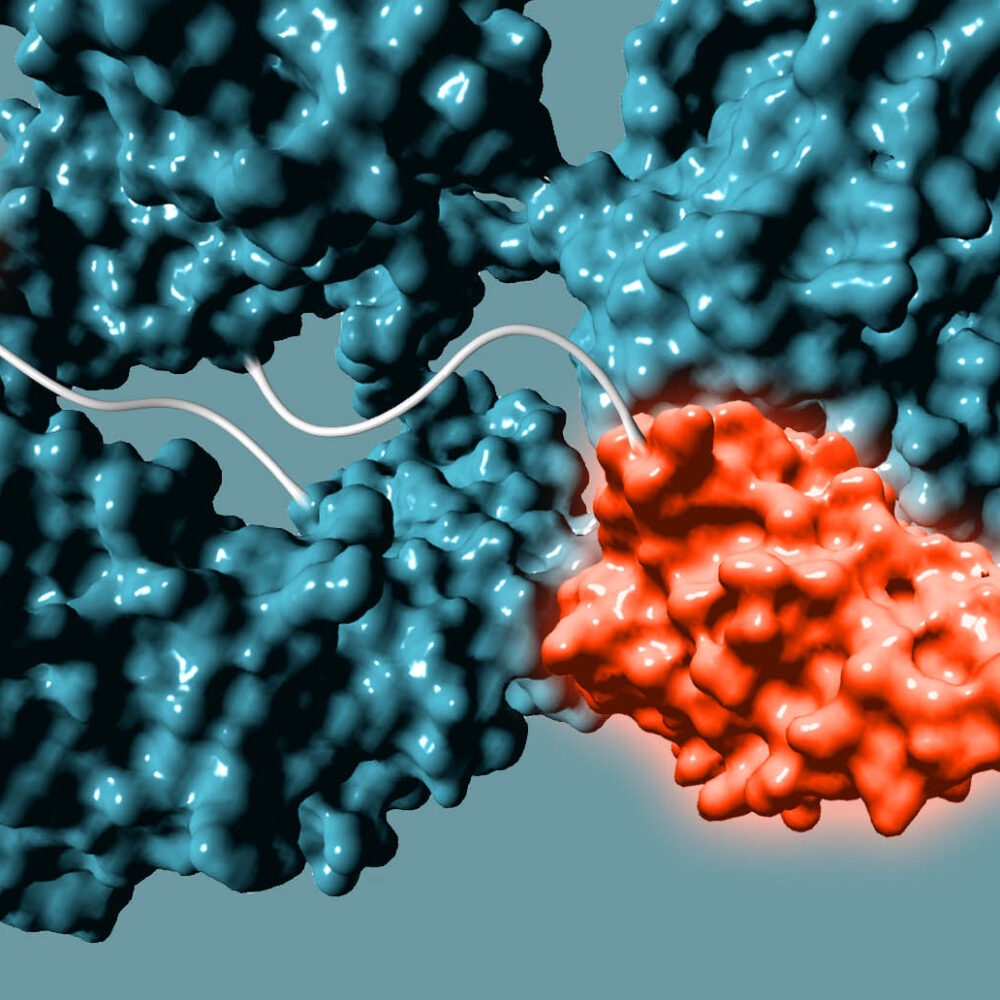
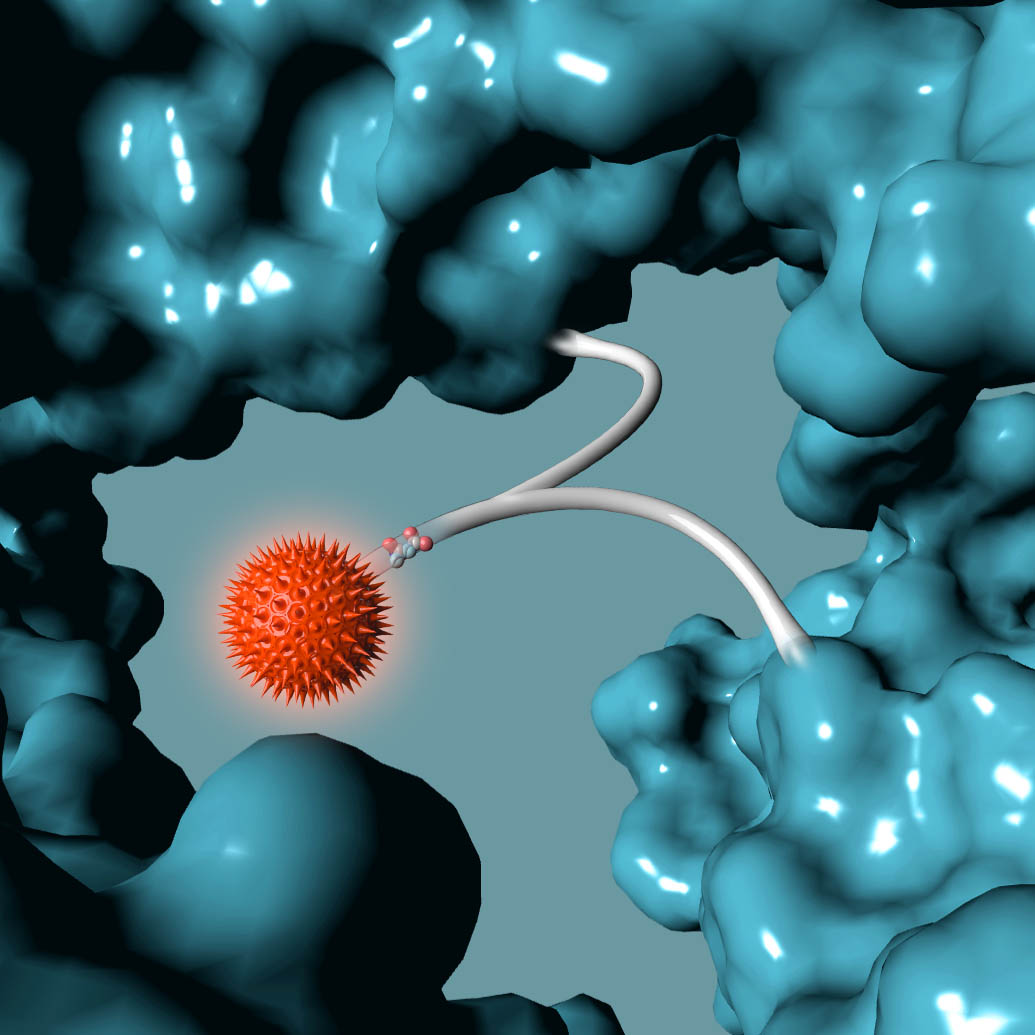
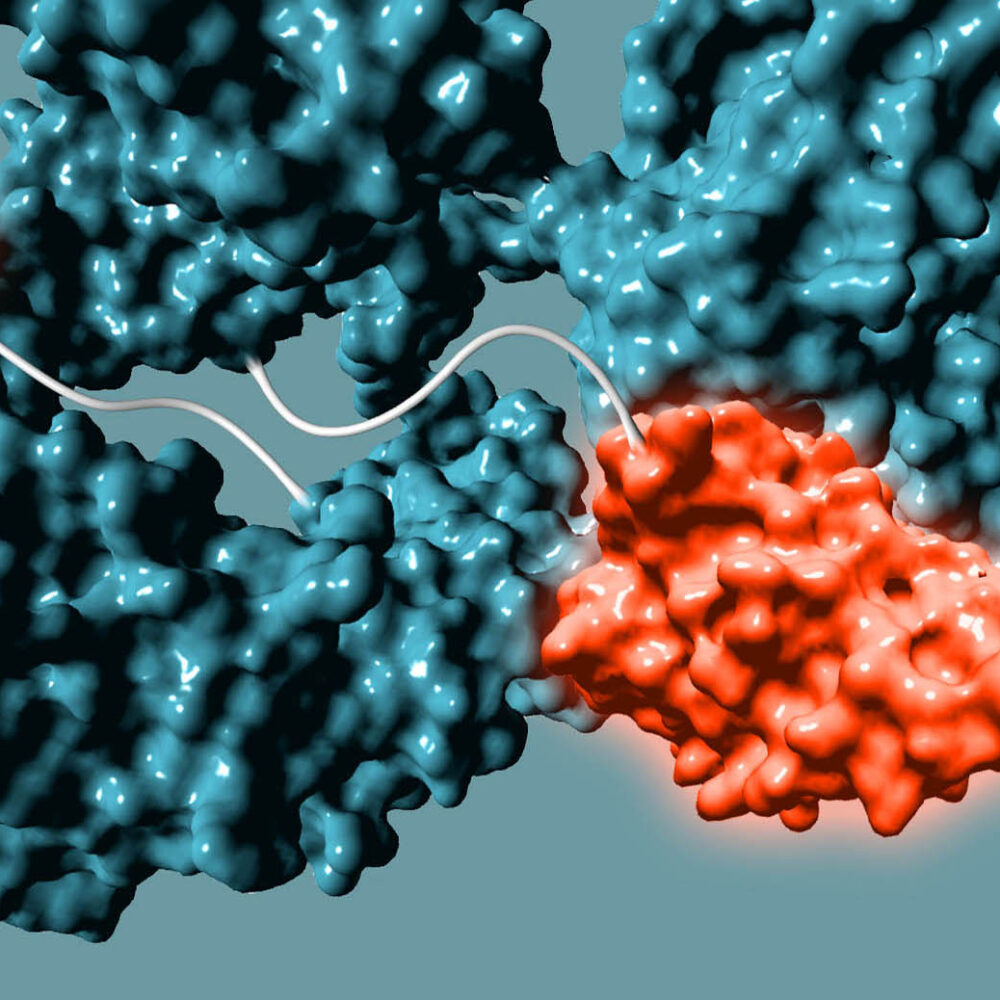
Building from of the well-understood cellular targeting abilities of antibodies, we combine the intrinsic efficiency of enzymes with the unique specificity of metal-free click chemistry to replace the native antibody glycan with a stably attached therapeutic payload.
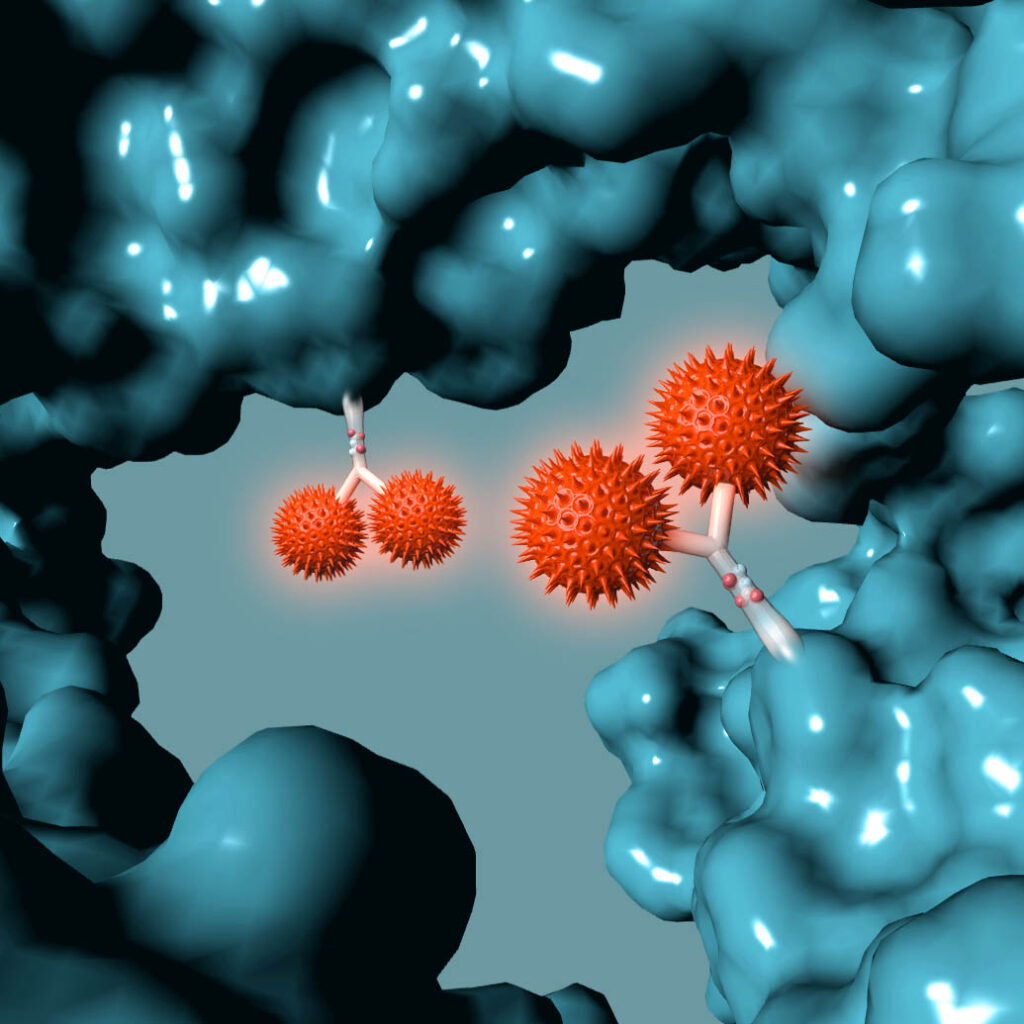
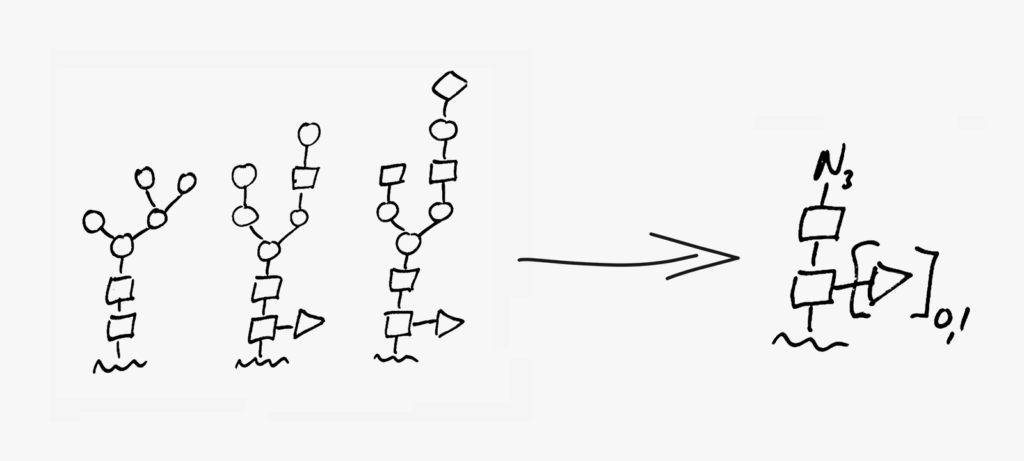
Following millions of years of evolution, the native glycan was retained in a similar position on all antibodies and GlycoConnect™ is built entirely around this.
Explore What's Possible
Antibody-Drug Conjugates
Enabling best-in-class, all under one roof
Technology & How We Differentiate
- GlycoConnect™ enables best-in-class therapeutic efficacy and tolerability
- HydraSpace™ further differentiates efficacy and tolerability versus other site-specific ADC approaches
- toxSYN™ linker-payloads provide multiple options to maximize efficacy in light of tumor biology
- Proprietary DAR1 technology
- Protected by worldwide patent estate, consisting of 25+ patents
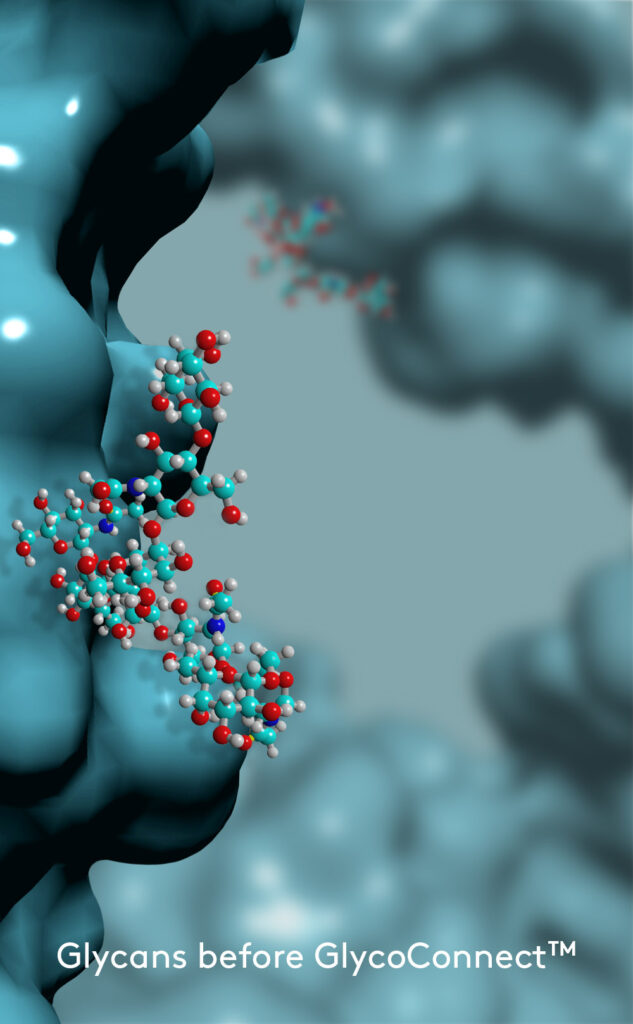
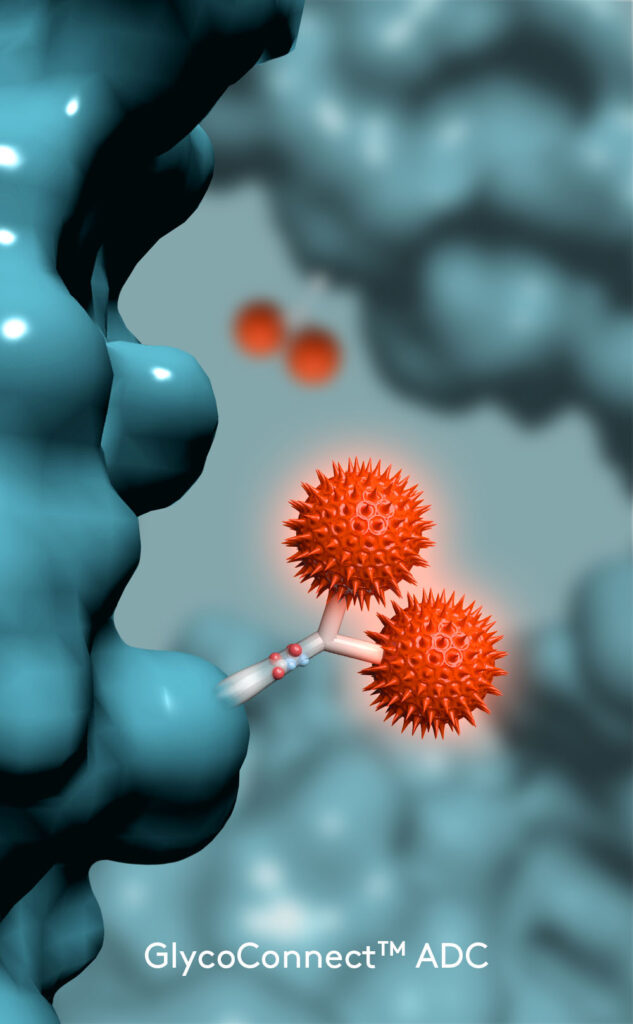
How It Works
GlycoConnect™ Antibody-Drug Conjugation
Any antibody can be converted into a stably conjugated ADC in just a few days by modifying the native antibody glycan using our highly efficient enzymes and metal-free click chemistry approach.
Multiple independent experiments confirm that the native glycan position is likely one of the best for attaching ADC payloads to antibodies.
Bispecifics
Enabling immune cell engagers & other protein conjugates
Technology & How We Differentiate
- Novel 2:1 format expected to deliver safety advantage
- Avoid co-expressing a genetic fusion of two separate antibodies / binding domains
- Directly convert any off-the-shelf antibody into a bispecific
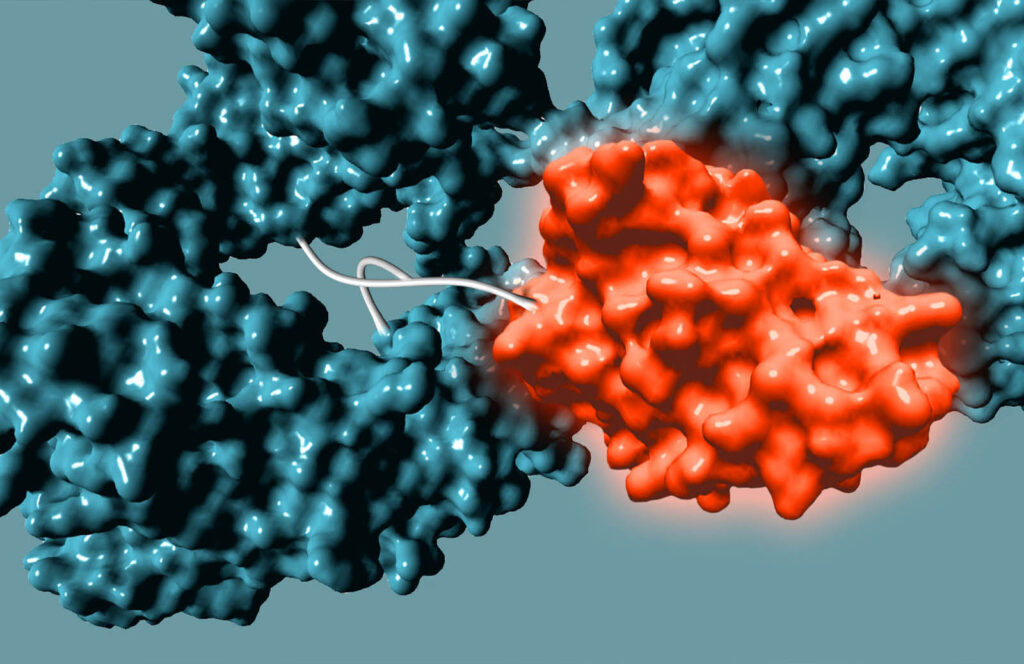
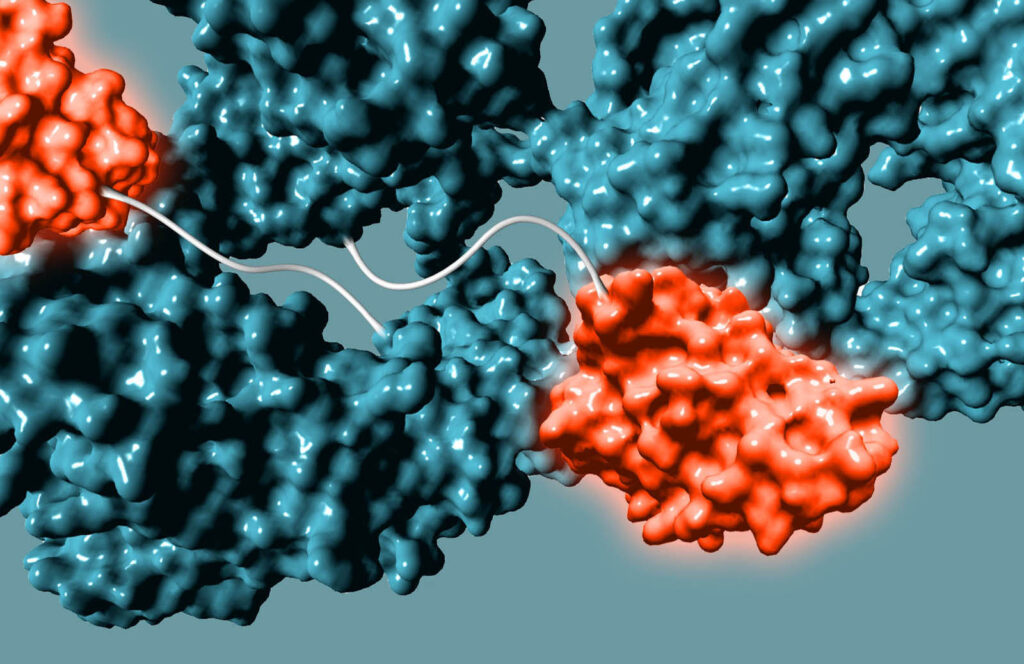
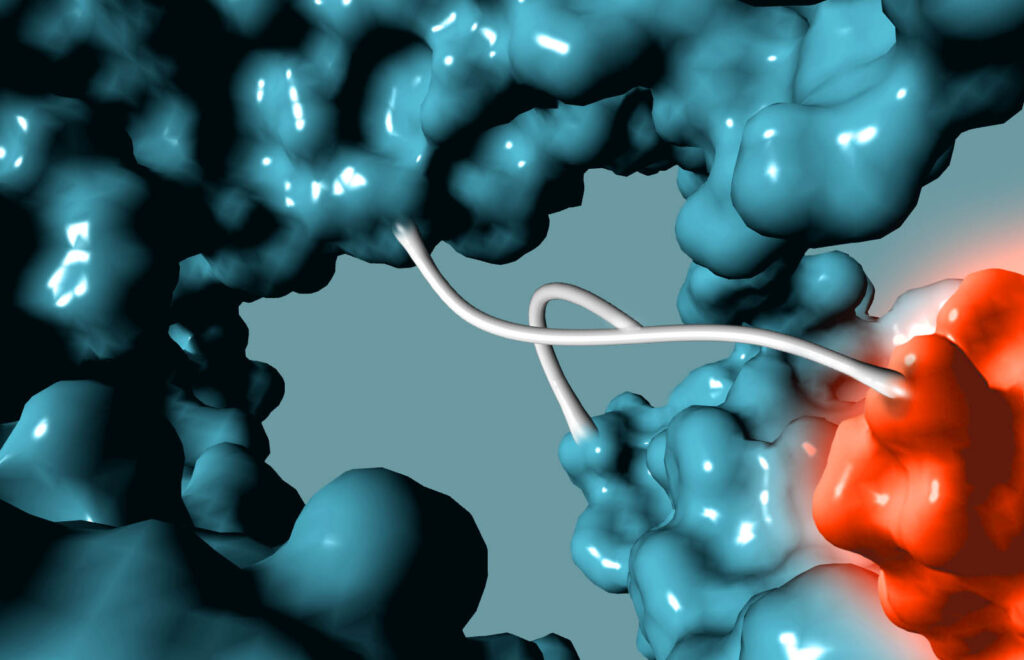
How It Works
Non-genetic Approach to Bispecifics
We use GlycoConnect™ to conjugate a separate antibody binding domain or cytokine (similar to an ADC payload), enabling bispecific immune cell engagers or checkpoint inhibitors from full-sized antibodies.