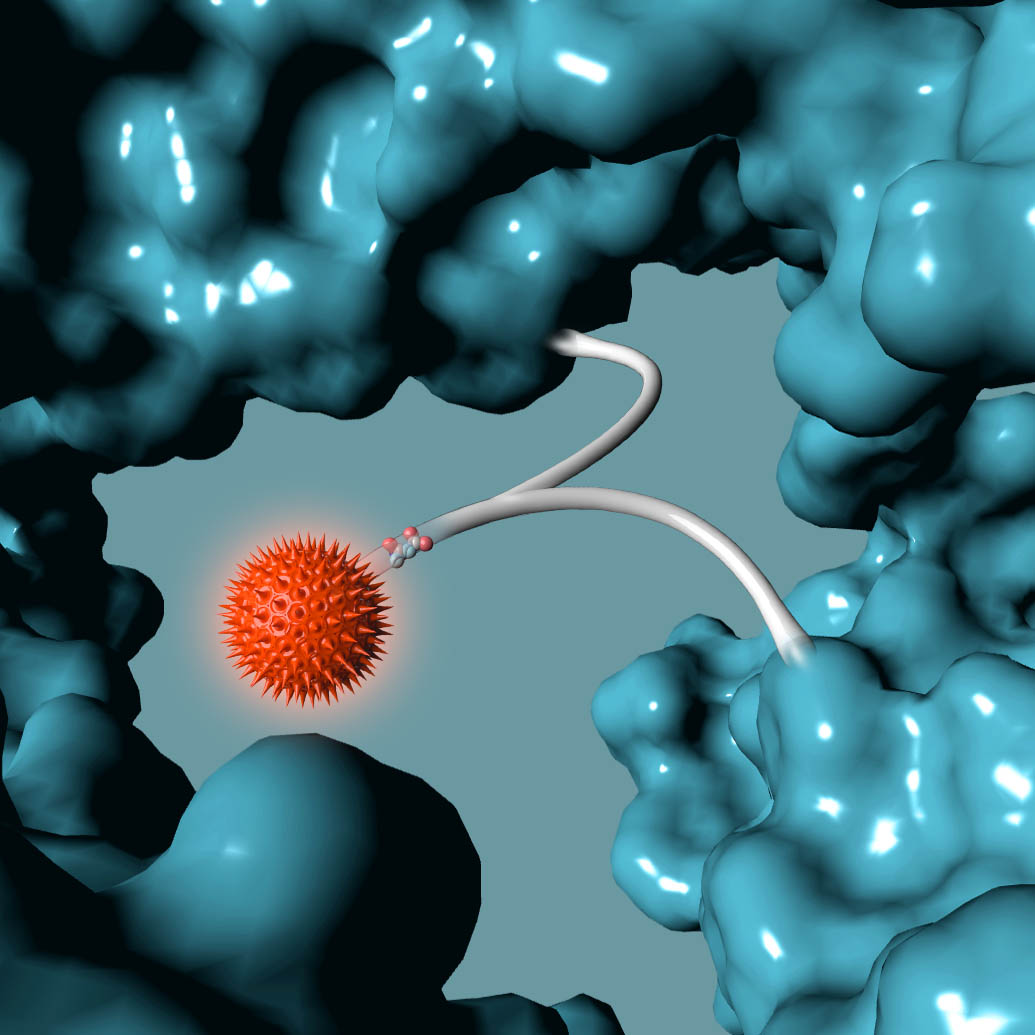
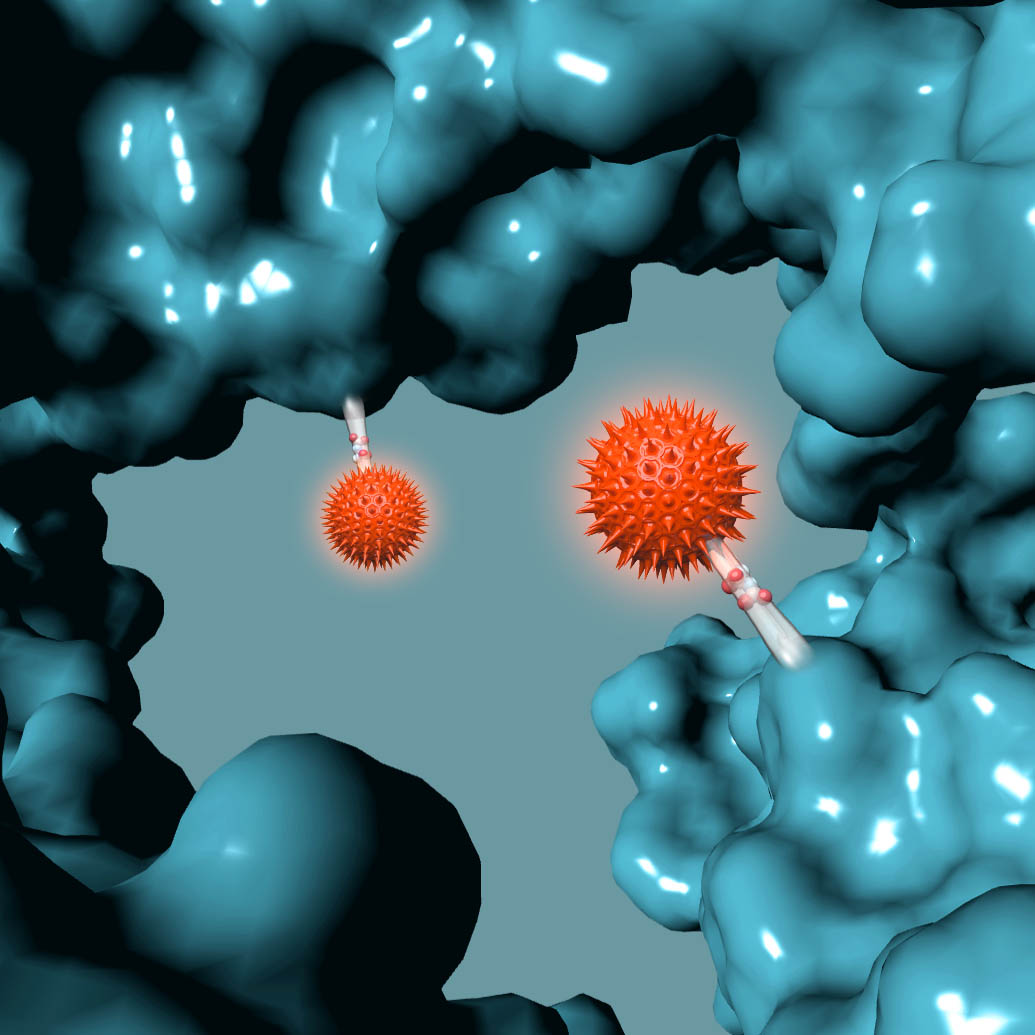
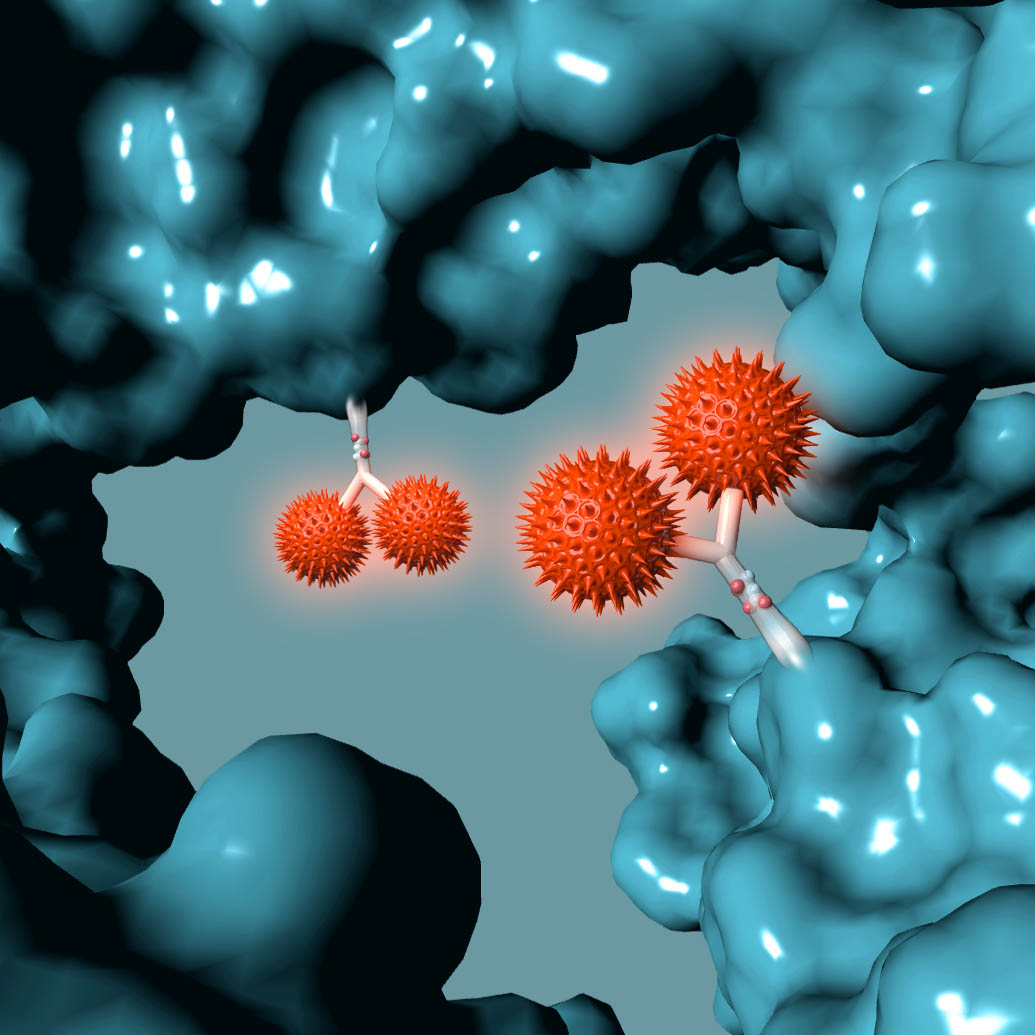
AMSTERDAM, NETHERLANDS – Synaffix BV, a biotechnology company exclusively focused on the development of industry-leading antibody-drug conjugate (ADC) technology platforms, today announced that a key patent that encompasses the entire GlycoConnect™ process used to prepare glycan-conjugated ADCs has been granted in the US (US 9,504,758 B2), further strengthening its IP portfolio.
The patent titled “MODIFIED ANTIBODY, ANTIBODY-CONJUGATE AND PROCESS FOR THE PREPARATION THEREOF”, covers key aspects of the GlycoConnect™ site-specific ADC platform that is based on attaching payloads directly to the antibody glycan without the need for prior protein engineering. The process utilizes two highly efficient enzymes together with best-in-class metal-free click chemistry and results in ADCs with a significantly expanded therapeutic index. The superiority of this approach has been confirmed in head-to-head comparisons to both FDA-approved ADC products, as well as to ADCs that have been obtained using other mainstream site-specific conjugation approaches.
“The granting of this patent represents a key milestone in the evolution of our patent portfolio and highlights the strength of our technology,” said Floris van Delft, CSO of Synaffix. “This patent, together with our granted patent on copper-free click chemistry and the patent applications that cover HydraSpaceTM, will serve an important role for all future drugs developed using our glycan-based ADC technology.”
About GlycoConnect™ and HydraSpace™ Platforms
The Synaffix technology platforms include GlycoConnect™, the site-specific and stable antibody conjugation technology that involves proprietary enzymes and metal-free click conjugation reagents, and HydraSpace™, the ADC-enhancing spacer technology. GlycoConnect™ was shown to be capable of significantly enhancing the therapeutic index of an ADC on its own. The highly polar properties of HydraSpace™ improve the solubility and stability of the payload and the resulting ADC product, thus enhancing further the therapeutic index of the ADC. Both technologies have demonstrated compatibility with all ADC payload classes and all IgG isotypes without requiring antibody engineering.
About Synaffix BV
Synaffix BV is a Netherlands-based biotechnology company exclusively focused on the continued advancement of best-in-class ADC technology platforms.
As a leading innovator in the field of ADCs offering absolute versatility and state-of-the-art solutions, our vision is to become the preferred partner in the development of these complex biological therapeutics and realize our ambition – connect to cure™.
Synaffix is backed by a top tier, life science-focused investor syndicate including Aravis, BioGeneration Ventures, BOM Capital and Merck Ventures, the strategic corporate venture capital fund of Merck.
Synaffix BV Contact